1、Dual-optical surface Semi-lOL
Due to the innovative design of the formulation, UV absorbers and blue filter compounds, we have fabricated a series of IOLs with good mechanical properties, good biocompatibility and less Glistening (almost none), which have successfully passed the biological compatibility test carried out by testing institute of medical device.
Dual-Optic and 360° square edge were formed using molding technology, thus the production of aspheric intraocular lenses no longer rely on expensive single-point diamond processing equipment, which greatly reduce the manufacturer' S equipment investment, substantially increase product yield, and make quality control more simple and easier.

2、Specifications of Semi-lOL
(1)SO model: foldable hydrophobic acrylic materials
●High transparency, index of refraction>1.53
●Ultraviolet- blocking
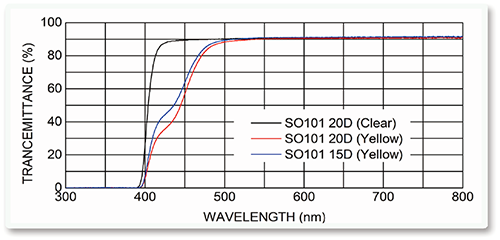
●Blue-light fitering(optional)Glistening less (almost none)
●Excellent biocompatibility
" Characteristic: WithoutGlistening, No adhesion of haptic during injection".

(2)HPRS model: foldable hydrophobic acrylic materials
●High transparency, index of refraction>1.55
●Ultraviolet-blocking
●Blue-light filtering (optional)
●Glistening less Excellent biocompatibility

UV-blockclear hydrophobic Semi-IOL

UV-blockyellow hydrophobic Semi-IOL
1、Dual-optical surface Semi-lO
Due to the innovative design of the formulation, UV absorbers and blue filter compounds, we have fabricated a series of IOLs with good mechanical properties, good biocompatibility and less Glistening (almost none), which have successfully passed the biological compatibility test carried out by testing institute of medical device.
Dual-Optic and 360d° square edge were formed using molding technology, thus the production of aspheric intraocular lenses no longer rely on expensive single-point diamond processing equipment, which greatly reduce the manufacturer' S equipment investment, substantially increase product yield, and make quality control more simple and easier.

2、Specifications of Semi-lO
(1)SO model: foldable hydrophobic acrylic materials
●High transparency, index of refraction>1.53
●Ultraviolet- blocking
●Blue-light fitering(optional)Glistening less (almost none)
●Excellent biocompatibility
" Characteristic: WithoutGlistening, No adhesion of haptic during injection".


(2)HPRS model: foldable hydrophobic acrylic materials
●High transparency, index of refraction>1.55
●Ultraviolet-blocking
●Blue-light filtering (optional)
●Glistening less Excellent biocompatibility

UV-blockclear hydrophobic Semi-IOL

UV-blockyellow hydrophobic Semi-IOL
● The customized design of lOL and Semi-finished IOL (e.g. the power range) ;
●The guidance of factory construction andquality system according to manufacture process and regulatory;
●Counseling and guidanceservices for IOL producing;
●Give suggestion on facilities selection, provide the accessoriesand fittings for haptic milling.
1、Both aspheric surfaces are moulded.Under development,come soon.
1、Dual-optical surface Semi-IOL
Due to the innovative design of the formulation, uv absorbers and blue filter compounds, we have fabricated a series of lOLs with good mechanical properties, good biocompatbility and less Glistening (almost none), which have successfully passed the biological compatibility test carried out by testing institute of medical device.
Dual-Optic and 360° square edge were formed using molding technology, thus the production of aspheric intraocular lenses no longer rely on expensive single-point diamond processing equipment, which greatly reduce the manufacturer's equipment investment, substantially increase product yield,and make quality control more simple and easier.

2、Specifications of Semi-lOL
(1) SO model: foldable hydrophobic acrylic materials
●High transparency, index of refraction>1.53
●Ultraviolet- blocking
●Blue-light filtering(optional)
●Glistening less (almost none)
●Excellent biocompatibility
Characteristic: Without Glistening, No adhesion of haptic during injection;


(2)HPRS model: foldable hydrophobic acrylic materials
●High transparency, index of refraction>1.55
●Ultraviolet-blocking
●Blue-light filtering (optional)
●Glistening less
●Excellent biocompatibility

UV-block clear hydrophobic Semi-IOL
UV-block yellow hydrophobic Semi-IOL

Close cooperation between all departments and motivated employees are the basis for the successful development of our drugs.
The HEC Pharm research and development center has created a unique atmosphere and structure.
The Research and Development team is directed by global experts in the field of medicine, including specialists in the fields of chemistry, biology and toxicology to mention a few. And they lead some of the most talented researched areas. Our team consists of 1,500 highly qualified employees, with more than 65% holding master’s or doctoral degrees. Our research team is rounded out by 16 world renowned experts and 22 PhDs. This contributes substantially to our innovations and success.

Our high degree of success if also based on HEC Pham’s outstanding experimental facilities. The Research and Development Center has a laboratory area covering more than 40,000 square meters and includes labs that strictly comply with Good Laboratory Procedures. High quality medicines are developed using the finest hi-tech medical equipment available in the world. In addition, we utilize advanced data base resources such as SciFinder, Reaxys, Thomson Pharma and Derwent World Patents Index.
Our high degree of success if also based on HEC Pham’s outstanding experimental facilities. The Research and Development Center has a laboratory area covering more than 40,000 square meters and includes labs that strictly comply with Good Laboratory Procedures. High quality medicines are developed using the finest hi-tech medical equipment available in the world. In addition, we utilize advanced data base resources such as SciFinder, Reaxys, Thomson Pharma and Derwent World Patents Index.
备案号:粤ICP备2021116245号-2 东阳光药 版权所有 互联网药品信息服务资格证书(粤)—非经营性—2020—0369